Announcement of the Ministry of Agriculture and Rural Affairs (No. 560)
In accordance with “Regulations on Administration of Veterinary Drugs” and the ” Measures for Registration of Veterinary Drugs”, the “Ministry of Agriculture and Rural Affairs (MARA)” examined and approved the re-registration of the veterinary drug, Tolfenamic acid Tablet produced by Vetoquinol S.A. The MARA issued “the Registration Certificate for Imported Veterinary Drugs” and published the revised product quality standards, manuals,d label, and process planning, which will be put into effect on the day of the newly issued of " the Registration Certificate of Imported Veterinary Drugs”. The previously issued product quality standards, manuals and label shall be repealed simultaneously.
The MARA approved the re-registration of two vaccines,including the swine atrophic rhinitis inactivated vaccine, produced by Zoetis' Lincoln Manufacturing Plant, United States and another enterprise. The MARA issued the “Registration Certificate for Imported Veterinary Drugs” and published the revised product quality standards, manuals and label, which will be put into effect on the day of the newly issued of " the Registration Certificate of Imported Veterinary Drugs”. The two kinds of previously issued veterinary drugs product quality standards, manuals and label shall be repealed simultaneously.
The MARA approved the change of registration of two kinds of veterinary drugs including the swine atrophic rhinitis inactivated vaccine produced by United Kingdom production plant of Elanco Animal Health Incorporated, United States and another enterprise.
The announcement was hereby published.
Attachment:
1. The Catalogue for Registration of Imported Veterinary Drugs
2. Product Quality standards
3. Manuals and labels
4. Process Planning
Ministry of Agriculture and Rural Affairs
26 May,2022
Attachmen 1: The Catalogue for Registration of Imported Veterinary Drugs
| List of Veterinary Drugs | Factory name |
Country | Imported veterinary drugs Registration certificate number | Validity period | Remark |
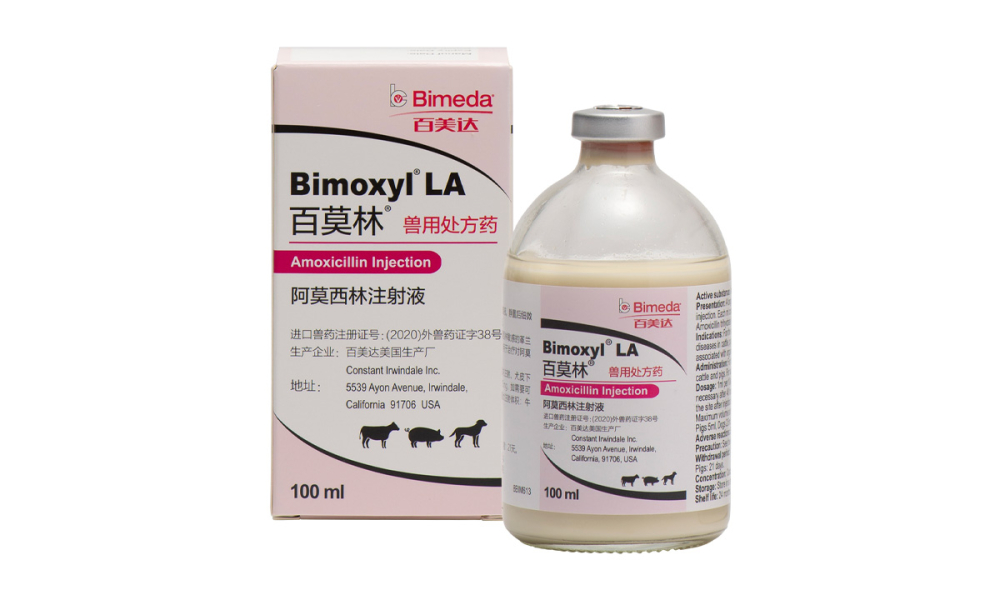
| ( 100ml : 15g ) Amoxicillin Injection |
Bimeda US production plant Constant Irwindale Inc. |
U.S. | ( 2020 ) Imported veterinary drug registration certificate No. 38 |
2020.09.07 — 2025.09.06 |
Change of registration: the validity period is changed to 36 months |
| ( 250ml : 37.5g ) Amoxicillin Injection |
( 2020 ) Imported veterinary drug registration certificate No. 39 |
Disclaimer:
This document in Chinese has been translated from English in order to reach a wider audience. However, Chinese is the official language of the announcement and the Chinese original of this document is the only authentic (that is, official and authoritative) text. Any citations must refer to the Chinese original of these documents.